Ansell Healthcare Products, LLC is voluntarily recalling 1,312 cases of MICROFLEX® Diamond Grip MF-300 examination gloves with Lot Number 2003LG, Batch Number 20035314LG and Manufacturing Date 2020-03-28 which were shipped between July 27 - August 10, 2020. After arriving at our warehouse, it was shipped prematurely, before FDA-required third-party testing had been completed to verify barrier integrity. As a result, Ansell is voluntarily recalling this product.
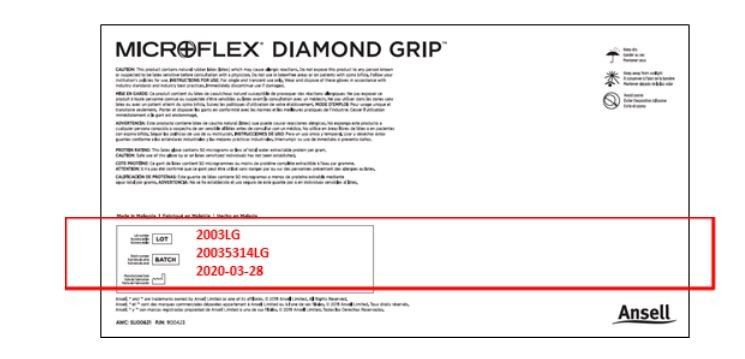
Customers can identify whether the MICROFLEX Diamond Grip MF-300 they received is part of this voluntary recall by looking for Lot Number 2003LG, Batch Number 20035314LG and Manufacturing Date 2020-03-28 as pictured below.
On Case Shipping Labels:
On the Bottom Panel of Dispenser Boxes:
If you believe you may have received MICROFLEX® Diamond Grip MF-300 examination gloves with these Lot, Batch and Manufacturing Date numbers, please visit: http://www.novasyte.com/ansell/capa-206-2020 to complete the necessary acknowledgement form. Upon completion of the acknowledgement form, Ansell will partner with you to arrange for a return of the product, provide a credit, and offer you the option of a reship. For any assistance regarding the acknowledgement form, please contact Novasyte at 855-863-7288 or ansell-capa-206-2020@novasyte.com.