As of December 19, 2016, the FDA published the final rule to ban powdered surgical gloves, powdered patient examination gloves, and absorbable powder used for lubricating surgical gloves. The FDA ruled these products present unreasonable and substantial risk to health care providers, patients and other individuals.
In response to the FDA ban on US powdered surgical gloves, powdered patient examination gloves, and absorbable powder used for lubricating surgical gloves, Ansell, a global leader in protection solutions, is committed to helping its valued customers manage the process of converting to appropriate alternatives.
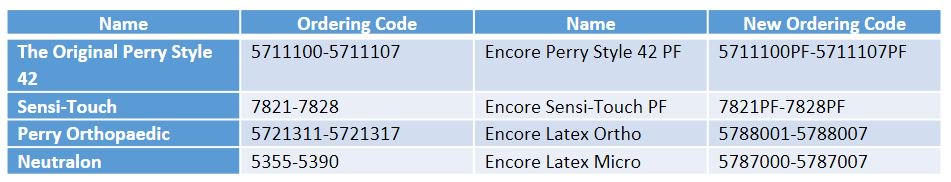
For surgical gloves we suggest the following substitutions, please note the new ordering codes: